Explosion and Flammability Ranges
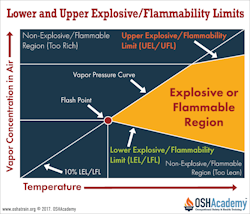
The lower explosive limit (LEL) or lower flammable limit (LFL) of a substance is the minimum concentration of gas or vapor in air below which the substance will not burn when exposed to a source of ignition.
This concentration is usually expressed in percent by volume. Below this concentration, the mixture is too "lean" to burn or explode.
The upper explosive limit (UEL) or upper flammable limit (UFL) of a substance is the maximum concentration of gas or vapor above which the substance will not burn when exposed to a source of ignition. Above this concentration, the mixture is too "rich" to burn or explode.
The flammable range is the range of concentrations between the LFL and UFL where the gas-air mixture will support combustion.
The flashpoint of a substance is the minimum temperature at which it gives off sufficient vapor to form an ignitable mixture with the air just above the surface of the substance. Ignition of a substance at the flashpoint is not continuous.
The ignition temperature or autoignition temperature is the minimum temperature required to initiate or cause self-sustained combustion without an ignition source.
When evaluating the fire or explosion potential at a hazardous waste site, all equipment used should be intrinsically safe or explosion-proof.
LEL Bottle Rocket Demonstration
Where flammable or explosive atmospheres are detected, ventilation may dilute the mixture to below the LEL/LFL. However, ventilation is generally not recommended if concentrations exceed the UFL/UEL, since the mixture will pass through the flammable/explosive range as it is diluted. Note that combustible gas indicator readings may not be accurate when oxygen concentrations are less than 19.5 percent.
Knowledge Check Choose the best answer for the question.
3-5. What is the range of concentrations between the LFL and UFL where the gas-air mixture will support combustion?
You forgot to answer the question!